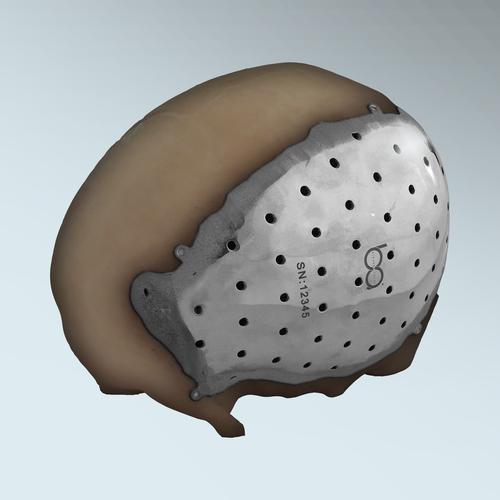
The FDA (Food & Drug Administration) has approved a 3D-printed, titanium, cranial/craniofacial patient-specific plate implant for use in the US. The implant was designed by Brazil-based BioArchitects, and is 3D printed using Sweden-based Arcam’s electron beam melting (EBM) process.
According to Design News this is the first 3D-printed implanused in the US. The design takes advantage of the light weight and tensile strength of a biocompatible titanium alloy.
Read the full story at Design News
Share This:
